Pharmaceutical price regulation has been used in major pharmaceutical markets to correct market imperfections and contain rising healthcare costs. But regulations can affect the adoption of innovations, especially in highly regulated industries such as the pharmaceutical industry where products and processes are protected by intellectual property rights. Lags in adoption of new pharmaceutical innovations may affect consumer welfare through impaired access to new drug products, in particular cost-effective products.
Thus, price regulation is contentious. There is some evidence to suggest that price regulation can delay launch of new drugs. This delay stems from the inevitable price and reimbursement negotiation processes, with governments' and firms' strategies combining to cause delay or even a non-launch of new drugs in low-priced markets (Danzon et al. 2005, Kyle 2007). From a health-policy perspective, such delays and non-adoptions can compromise health outcomes and the quality of healthcare. It can also lead to dependency on older pharmaceutical molecules that exhibit lower therapeutic value or cost effectiveness (Danzon and Ketcham 2004, Schoffski 2002, Kessler 2004, Wertheimer and Santella 2004).
Prices and delays in international drug launches
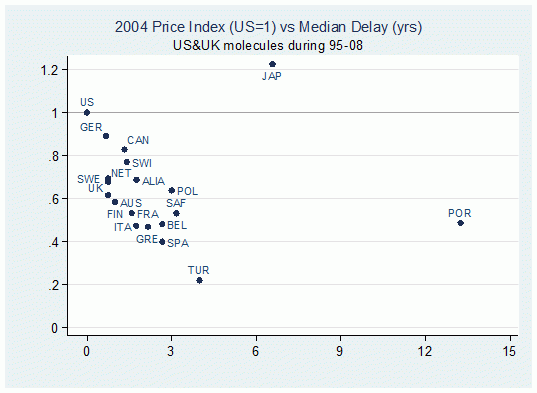
In a new study (Varol et al. 2011), we characterise patterns of delay in 20 major pharmaceutical markets for new molecules from 14 different therapeutic classes during 1960-1998. The delay for each molecule is calculated as the difference between the first global launch date and the corresponding local launch date in individual markets. Figure 1 illustrates the correlation between median delays and the bilateral price indexes in all 20 markets with respect to US prices in 2004 (the correlation is -0.47 and is significant at the 0.01 level). This suggests that indeed delay is longer in lower-priced markets.
Figure 1. Bilateral price indices with respect to US prices vs. median delays (for ethical branded products in the retail sector)
Note: Bilateral price indexes are calculated by considering common molecules in the US and the respective country, prices are weighted by the US volume.1
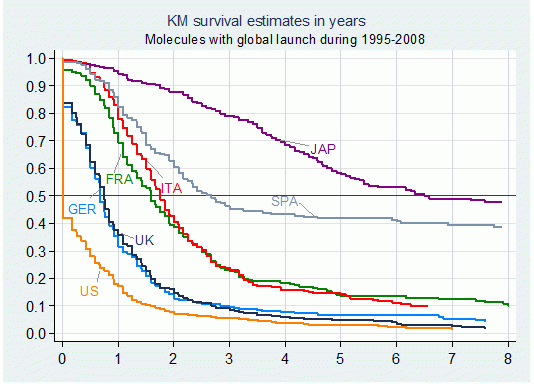
Figure 2 illustrates the pattern of delay for the biggest seven price-regulated markets. In Italy, France and Spain, for example, introductions lag about 1.6 to 2.6 years behind free-priced markets (US, UK and Germany). The Japanese market exhibits a highly paradoxical behaviour with a substantial delay of 6.5 years. This anomaly results from the fact that the Japanese pharmaceutical industry remains predominantly domestic and is still uncompetitive.
Figure 2. Median Delays by country for molecules with global launch between 1995 and 2008
Note: Failure defined as launch in individual markets
Our regression analysis suggests that, even controlling for other factors, the main message from Figure 1 holds up: low prices have a statistically significant delay on molecule-country launches relative to the first global launch. Our findings can be summarised as follows:
- High expected prices increase the speed of cross-country diffusion of pharmaceutical innovation. Therefore, price regulations that reduce prices result in delayed access to pharmaceutical innovation.
- Pharmaceutical innovations with a higher expected market size diffuse internationally faster, controlling for the effect of expected price.
- Firms' economies of scale and scope increase the likelihood of launch.
- Therapeutically/commercially important molecules diffuse internationally faster.
Policy implications?
The net impact of price regulations on societal welfare is not clear.
- Society loses from delayed introductions of cost-effective innovations, but
- Gains static efficiency improvements from reducing mark ups over marginal cost.
Moreover, price controls have negative implications on dynamic efficiency by reducing incentives for (timely) entry and the extent of competition. Delays in adoption reduce the net present value of R&D investments by delaying cash flows and shortening the exclusivity period, which has been observed to reduce future R&D outlays and innovation. This may be overcome by new innovation models (i.e. open innovation) and reward mechanisms other than patents.
References
Danzon, PM and A Epstein (2005), “Launch and Pricing Strategies of Pharmaceuticals in Interdependent Markets”, iHEA Conference.
Danzon, PM and JD Ketcham (2004), “Reference Pricing of Pharmaceuticals for Medicare: Evidence from Germany, the Netherlands, and New Zealand” in DM Cutler and AM Garber (eds.), Frontiers in Health Policy Research, Volume 7, MIT Press,1-54.
Danzon, PM, Y Wang, And L Wang (2005), "The Impact of Price Regulation on the Launch Delay of New Drugs - Evidence from Twenty-Five Major Markets in the 1990s", Health Economics, 14:269-292.
Kessler, DP (2004), "The Effects of Pharmaceutical Price Controls on the Cost and Quality of Medical Care: A Review of the Empirical Literature", ita.doc.gov.
Kyle, M (2007), "Pharmaceutical Price Controls and Entry Strategies", The Review of Economics and Statistics, 89(1):88-99.
Varol, Nebibe, Joan Costa-i-Font, Alistair McGuire (2011), “Explaining Early Adoption on New Medicines: Regulation, Innovation and Scale”, CESIfo Working Paper Series, 3459.
Wertheimer, AI and TM Santella (2004), "Pharmacoevolution: the advantages of incremental innovation", IPN Working Papers on Intellectual Property, Innovation and Health.
1 ALIA: Australia; AUS: Austria; CAN: Canada; FIN: Finland; FRA: France; GER: Germany; GRE: Greece; ITA: Italy; JAP: Japan; NET: Netherlands; POL: Poland; POR: Portugal; TUR: Turkey; SAF: South Africa; SPA: Spain; SWE: Sweden; SWI: Switzerland