The public health literature has long studied the drivers of vaccination choices. The coronavirus pandemic has made the reasons that lead people to refuse immunisation a central issue for policymaking (e.g. Blanchard-Rohner et al. 2021, Costa-i-Font 2021, Campos-Mercade et al. 2021). Economic reasoning explains the decision to get the vaccine as a rational choice between two perceived risks: contracting the disease and experiencing vaccine side-effects (e.g. Binder and Nuscheler 2017, Bohm et al. 2016, Chang et al. 2021).
Empirical studies have shown that media coverage of controversies about vaccine safety significantly encourages vaccine refusal, probably because it leads people to overestimate the incidence and severity of adverse events. For example, reports of the MMR-autism controversy led to a decline in the vaccination rate, with negative spillovers onto other vaccines (Carrieri et al. 2019, Chang 2017).
New study on the COVID immunisation campaign in Italy
But what happens when doubts about vaccine safety arise from health authorities? In a new paper (Deiana et al. 2022), we exploit a quasi-experiment arising from the suspension of the ChAdOx1-S vaccine – initially called AstraZeneca and later rebranded as Vaxzevria (VA) – to study the drivers of vaccine hesitancy (defined as the refusal or delay of vaccination despite the availability of vaccination services).
In Italy, the National Medicines Agency (AIFA) interrupted the inoculations of a VA batch on 11 March 2021, following reports of very rare thrombotic thrombocytopenia among vaccine recipients. On 15 March, the AIFA suspended all VA vaccinations for four days to examine the potential size and severity of the adverse events while allowing Pfizer-Biontech (PB) injections to continue. We take advantage of the quasi-experimental setting, which arose from the health authorities’ decision, to explore the potential drivers of vaccine hesitancy. Exploiting a difference-in-differences design whereby the VA vaccine is the treatment group and the PB vaccine serves as the control group, we start by quantifying vaccine hesitancy in Italy during the first stage of the immunisation campaign and then dig into the potential drivers of vaccination refusal.
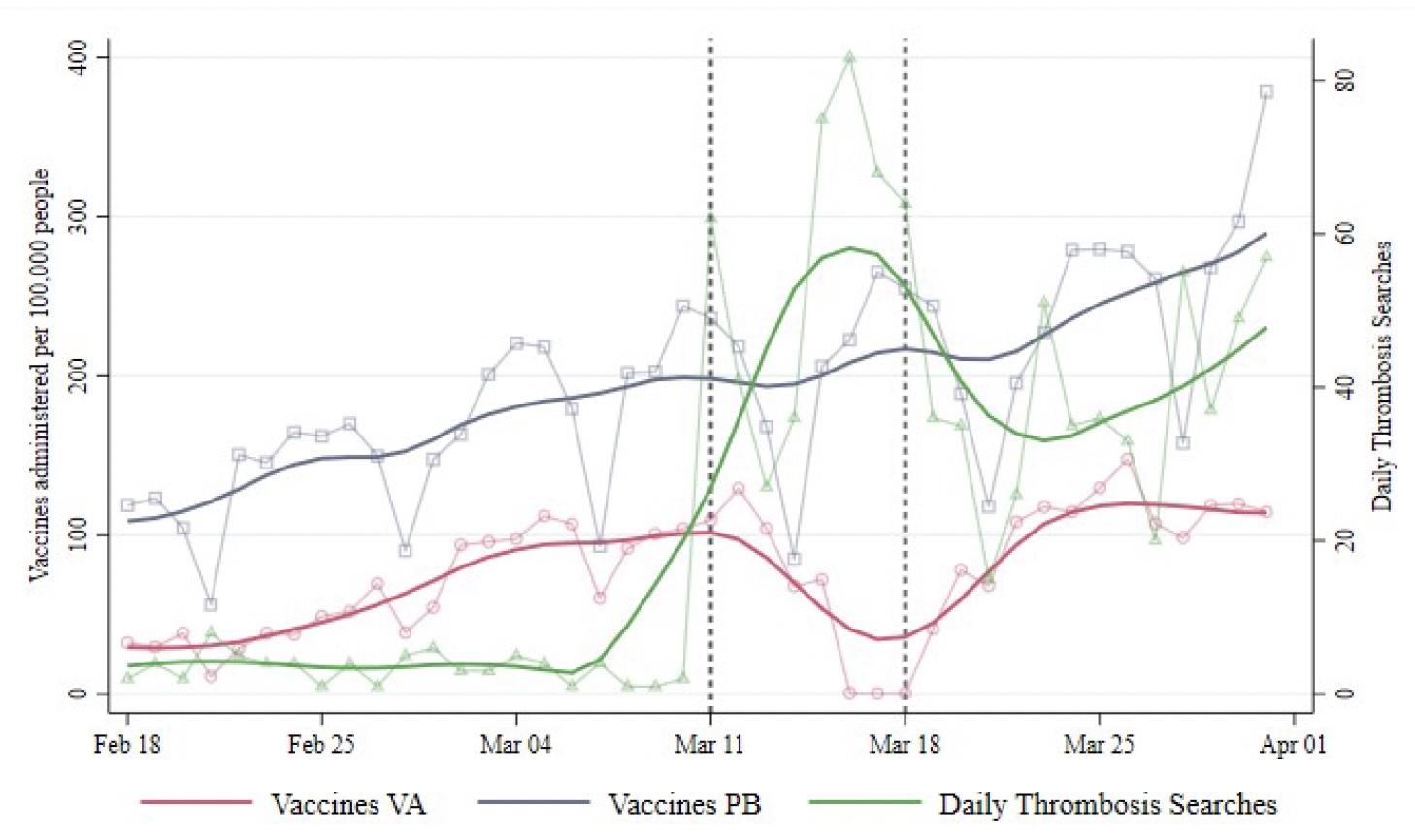
Figure 1 illustrates the daily total number of doses administered in Italy per 100,000 inhabitants. The solid red and blue lines describe the trends in VA and PB injections, respectively. The green line represents the trend in daily online searches for the word “thrombosis”, which peaked during the suspension week. The inoculation rates for the two vaccines followed parallel patterns until the suspension of the first VA batch on 11 March. In the suspension week, when the hubs did not administer the vaccine for four days, the VA vaccination rate decreased by roughly 46 daily doses per 100,000 inhabitants. The resulting value was approximately 60% lower than the level that would have followed from the pre-event pattern.
Figure 1 Administered vaccines and searches for “thrombosis”
After the campaign resumed on 19 March, the daily rate of VA inoculations declined further. The authors observed a reduction of 63 daily doses per 100,000 inhabitants, implying a 55% lower vaccination rate. Two weeks later, the negative effect persisted, leading to a drop of 86 daily doses per 100,000 inhabitants, corresponding to a 58% lower level.
Balancing two perceived risks
To delve deeper into the possible drivers of hesitancy, we tested whether the response to the VA suspension varied with the severity of the outbreak and Google searches for “thrombosis” across Italian regions. The maps below show the geographic distribution of the search intensity for the term “thrombosis” from 11 to 18 March 2021 (on the left) and the cumulative number of COVID-19 cases per 100,000 people from the beginning of the pandemic until 11 March 2021.
Figure 2 displays the geographical distribution of the variables we use in the heterogeneity analysis to understand the drivers of vaccine hesitancy after the VA suspension.
Figure 2
After authorities resumed the VA roll-out, the gap in inoculation rates between the two vaccines shrank with local COVID cases and broadened in regions paying greater attention to vaccine-adverse events.
Overall, the findings suggest that the slowdown in the immunisation campaign was demand-driven. The four-day suspension caused a statistically significant reduction in VA injections that persisted weeks after authorities restored the immunisation campaign and reassured the public about the vaccine’s safety. The broadening gap between the two vaccines in regions displaying greater interest in “thrombosis” suggests that the VA suspension may have attracted the public’s attention to vaccine-adverse events related to VA injections, possibly inflating their risk. This evidence is in line with the literature on hesitancy that identifies concerns about the incidence and severity of adverse events as one of the main drivers of the decision to postpone or refuse vaccination. On the other hand, the narrowing gap between the two vaccines in regions suffering from higher COVID occurrence suggests that the severity of the outbreak mitigated the impact of candidate recipients’ concerns about the vaccine’s safety.
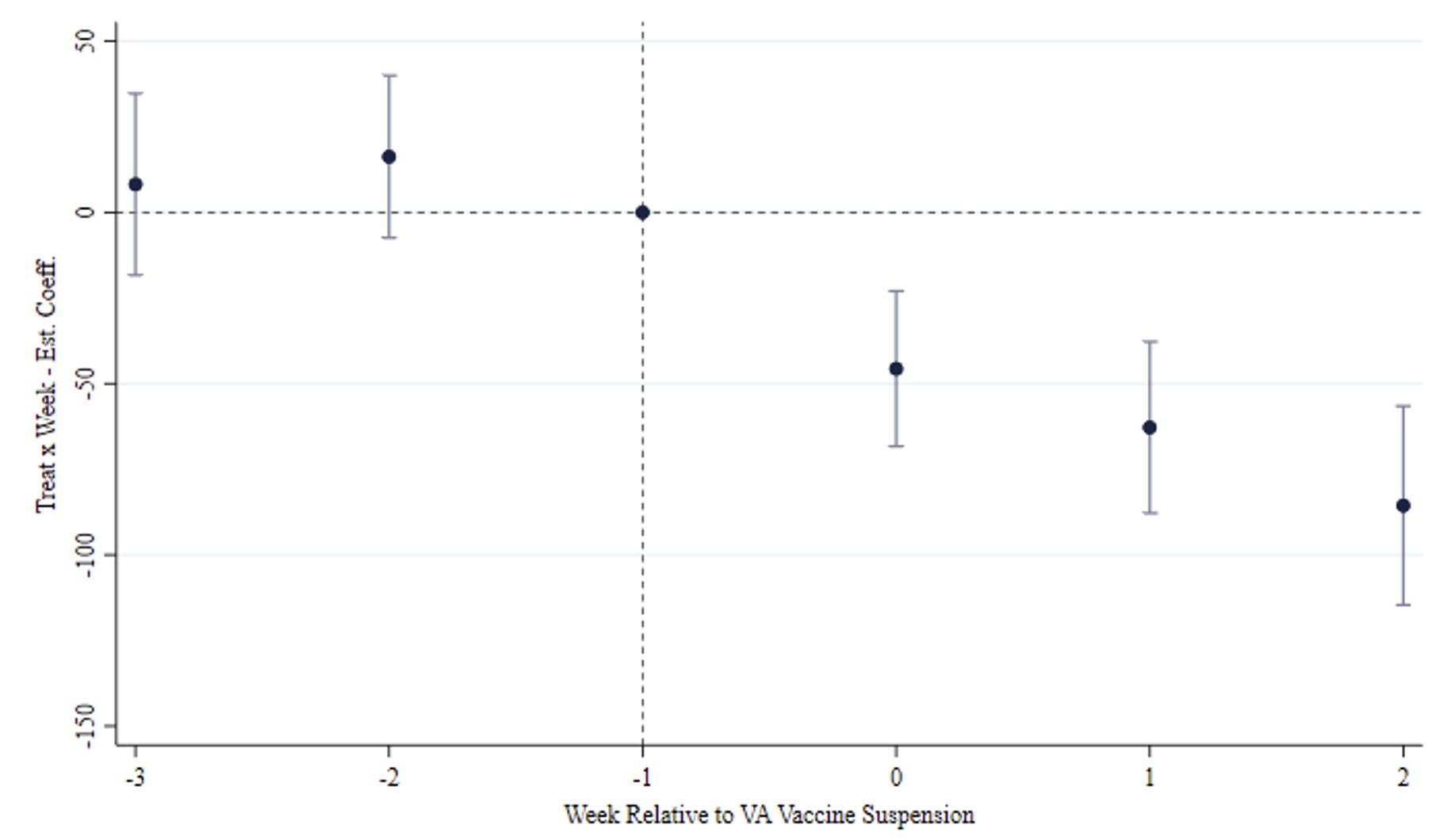
A daily event study shows there are no anticipation effects related to news reports of blood disorders among Vaxzevria recipients before the suspension. This evidence suggests that the spreading of alarming news about vaccine-adverse events did not prompt any statistically significant reaction in candidate recipients. Instead, it seems that people started to revise their assessment of the two risks informing vaccination decisions when doubts about the safety of the VA vaccine emerged from health authorities.
Figure 3 Event study of the main result
In summary, the suspension of the ChAdOx1-S vaccine may have unintentionally altered the balance between the perceived risks of contracting the disease and incurring vaccine-adverse events for many people, resulting in increased reluctance to get the vaccine. Altogether, our results reaffirm the importance of providing accurate institutional information about the relative incidence of the alternative risks that individuals weigh in their vaccination decisions.
Authors’ note: The views expressed are purely those of the authors and may not in any circumstances be regarded as stating an official position of the European Commission.
References
Binder, S and R Nuscheler (2017), “Risk-taking in vaccination, surgery, and gambling environments: Evidence from a framed laboratory experiment”, Health Economics 26(S3), 76–96.
Blanchard-Rohner, G, B Caprettini, D Rohner and H J Voth (2021), “From tragedy to hesitancy: How public health failures boosted COVID-19 vaccine scepticism”, VoxEU.org, 1 June.
Bohm, R, C Betsch and L Korn (2016), “Selfish-rational non-vaccination: Experimental evidence from an interactive vaccination game”, Journal of Economic Behavior and Organization 131: 183–195.
Campos-Mercade, P, A N Meir, F Schneider, S Meier, D Pope and E Wengström (2021), “Monetary incentives increase COVID-19 vaccinations, nudges do not”, VoxEU.org, 19 November.
Carrieri, V, L Madio and F Principe (2019), “Vaccine hesitancy and (fake) news: Quasi experimental evidence from Italy”, Health Economics 28: 1377–1382.
Chang, L V (2018), “Information, education, and health behaviors: Evidence from the MMR vaccine autism controversy”, Health Economics 27: 1043–1062.
Chang, T Y, M Jacobson, M Shah, R Pramanik and S B Shah (2021), “Financial incentives and other nudges do not increase COVID-19 vaccinations among the hesitant”, VoxEU.org, 8 December.
Costa-i-Font, J (2021), “Social value and incentives for vaccine uptake”, VoxEU.org, 29 June.
Deiana, C, A Geraci, G Mazzarella and F Sabatini (2022), “Perceived risk and vaccine hesitancy: Quasi-experimental evidence from Italy”, Health Economics.