Health and safety is of first-order importance in our lives. Product liability accounts for the majority of civil injury cases in the US, and so it is also critical for firms that produce the products. These accidents attract media coverage and public attention – for example, a fatal accident in 2018 involving an Uber autonomous vehicle, or the Boeing 737 Max crashes in Indonesia and Ethiopia in 2018 and 2019. The attention can have profound impacts on the perceived safety level of the underlying technologies.
If we decide that a product has become more risky, it may affect demand. This may also influence the rate and direction of technological progress (Viscusi 1993). On one hand, higher risk perception may suppress demand for a product class, reduce its market size and so chill R&D investments (Acemoglu and Linn 2004). On the other hand, higher willingness to pay for safer products may increase the incentives to innovate, and serve as a 'demand-pull' force for the development of risk-mitigating technologies (RMTs). These innovations reduce the probability of negative events or the severity of the consequences.
In 2009, there was extensive media coverage of over-radiation accidents involving CT scanners (Zarembo 2009). We exploited the increase in perception of the risk of medical devices using radiation to examine the impacts on firm innovation, and the development of RMTs (Galasso and Luo 2019).
Over-radiation accidents using CT scanners and the development of RMTs
On 8 October 2009, a medical centre in Los Angeles disclosed that it had administered up to eight times the normal radiation dose to more than 200 patients undergoing CT brain perfusion. The hospital had made a mistake in the scanner settings. These accidents were reported by both local and national media outlets. Most prominently, starting on 15 October, the New York Times published more than 20 articles on this and similar radiation accidents during the previous two years (Bogdanich 2009).
Congressional hearings, industry accounts, survey studies and discussions with practitioners, suggest that the media coverage significantly increased perceived risk of CT and other medical technologies using radiation among both patients and medical providers (Boutis et al. 2014). We examined the subsequent impacts on innovation using two types of measure:
- Patent applications filed at the US Patent and Trademarks Office. The detailed patent classification system allowed us to differentiate safety-related features from other features of radiation diagnostic devices.
- Pre-market notifications submitted to the FDA. Any manufacturer intending to market in the US must submit a notification for class-II 'moderate to high risk' devices, the class to which all medical diagnostic devices belong.
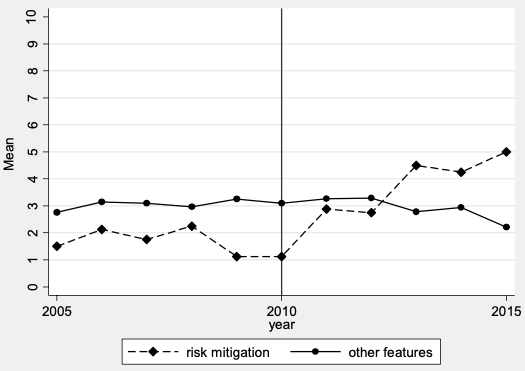
The year-specific patenting rates show that before the shock the average number of patent applications related to radiation diagnostic devices that were related to radiation safety – that is, protection against radiation, control of the level of radiation exposure, and detection of device malfunctions – was less than the average of other patent classes in these devices. After the shock, patenting activities for safety-related features increased, and the number of applications was higher than for other technology classes (Figure 1).
Figure 1 Patenting in RMT patent classes versus other classes in radiation diagnostic devices
Source: USPTO.
Notes: raw data. Average number of patents in risk-mitigating technology patent classes versus other classes in radiation diagnostic devices.
We can confirm this result with a series of difference-in-differences regressions that suggest a differential increase in RMT patenting after 2010 of more than 100%. The direction of innovation has changed, but this also suggests a net increase in the overall innovation activity in radiation diagnostic devices. In particular, FDA pre-market notification data shows the total number of new product introductions of radiation diagnostic devices after the shock increased by about 30%, relative to other medical devices that do not use radiation.1
RMTs may take various forms. They can be incremental innovations that refine existing technologies, or radical innovations that establish new, dominant designs (Utterback and Abernathy 1975, Dosi 1982). While many examples of RMTs are product innovations, process innovations – an assembly-line redesign to better identify defects, or the use of checklists during surgeries to reduce medical errors, for example – can also be RMTs.
Based on industry and clinical publications and the FDA application summary texts, we could characterise RMTs developed after the over-radiation shock into two types.
- Low-hanging fruit. Improvements to prevent overdose accidents. This type of RMTs – such as dose display, alert and notification systems for doses exceeding pre-assigned thresholds, standardised dose recording software, and redesigned use protocols for certain procedures – were mostly implemented through new standards set by the industry association.
- A new technique. These RMTs were qualitatively different. They required a substantial departure from the technique to reconstruct image data that had been dominant for 30 years. The industry quickly re-introduced and invested heavily in the development and marketing of a long-shelved technique that sacrificed speed and image quality, but reduced radiation doses by 80-90%, a level that could not be achieved by simply 'tweaking' the dominant method.
Increase in risk perception leads to increased demand for safety
Anecdotal and survey evidence connects the increase in innovation activities in radiation diagnostic devices, and the increase in risk perception following the media coverage of the over-radiation accidents. We found both a decline in usage, and an increase in equipment upgrades. In particular, Medicare data shows a decline of about 20% in the number of high-radiation imaging exams, relative to non-radiation exams such as MRI and ultrasound, after the shock.
At the same time, the FDA's X-ray Assembler data shows that the propensity of hospitals and clinics to replace or upgrade CT systems increased significantly after the shock, relative to equipment emitting lower levels of radiation.
Externalities from changes in risk perception may affect the entire product category. Consistent with this idea, a firm-level analysis suggests that innovation responses were industry-wide and came both from firms directly involved in the accidents and those that were not. In particular, large incumbents appear to have played an important role in the development and commercialisation of RMTs. The shock seems to have perpetuated, rather than diminished, their market dominance.
Liability risk and innovation
These findings contribute to our understanding of the relationship between liability risk and innovation. The dominant policy and legal view is that the liability system in the US chills willingness to develop new technologies (Porter 1990, for example). This has intuitive appeal, but there is little empirical evidence that links liability risk and innovation. Two large-sample empirical studies examining this issue – Viscusi and Moore (1993) and Galasso and Luo (2017) – show that, on average, higher liability risk induces higher R&D spending and more patenting.2 They suggested that this was likely due to the incentivising effect on the development of RMTs, but neither paper directly measured this type of innovation. Our paper specifically measures RMTs and provides direct evidence for the change in the direction of innovation.
The accidents in our empirical setting reflect a general problem, as machines have become increasingly complex. More tasks are automated in autonomous automobiles, robot-assisted surgeries, smart factories, and medical devices used in homes rather than hospitals. The ways in which humans interact with machines, and in which errors and misuse happen, may be difficult to predict.3
Revisions of liability systems (for example, re-allocating liability costs between users and producers of a product) to adapt to this increasingly automated world is being researched (e.g. Abraham and Rabin 2019, Shavell 2019), but is complex. Our research shows that risk perceptions of emerging technologies evolve with increasing use and, unfortunately, from accidents. the resulting changes in risk perception may lead to changes in technological development and industry standards, even when liability rules stay the same.
References
Abraham, K S, and R L Rabin (2019), "Automated Vehicles and Manufacturer Responsibility for Accidents: A New Legal Regime for a New Era", Virginia Law Review 105: 127-171.
Acemoglu, D and J Linn (2004), "Market Size in Innovation: Theory and Evidence from the Pharmaceutical Industry", Quarterly Journal of Economics 119: 1049-1090.
Bogdanich, W (2009), "Radiation Overdoses Point up Dangers of CT Scans", New York Times, 15 October.
Dosi, G (1982), "Technological paradigms and technological trajectories: a suggested interpretation of the determinants and directions of technical change", Research Policy 11: 147-162
Galasso, A and H Luo (2017), "Tort Reform and Innovation", Journal of Law and Economics 60: 385-412.
Galasso, A and H Luo (2018), "When does Product Liability Risk Chill Innovation? Evidence from Medical Implants", NBER working paper 25068.
Alberto Galasso, A and Hong Luo (2019), "Risk-Mitigating Technologies: the Case of Radiation Diagnostic Devices", CEPR discussion paper 13682.
Porter, M (1990), The competitive advantage of nations, Free Press.
Shavell, S (2019), "On the Redesign of Accident Liability for the World of Autonomous Vehicles", NBER working paper 26220.
Utterback, J and W J Abernathy (1975), "A Dynamic Model of Product and Process Innovation", Omega 3: 639-656.
Viscusi, K (1993), "The Value of Risks to Life and Health", Journal of Economic Literature 31: 1912-1946.
Viscusi, K and M Moore (1993), "Product liability, research and development, and innovation", Journal of Political Economy 101: 161-184.
Zarembo, A (2009), "Cedars-Sinai radiation overdoses went unseen at several points", Los Angeles Times, 14 October.
Endnotes
[1] Using textual information extracted from the FDA application summary files, we confirm that the increase was driven by products for which radiation safety features are prominent.
[2] Galasso and Luo (2018) found a negative impact of liability risk on innovation, but through an indirect mechanism: a large increase in the liability risk faced by upstream suppliers may result in supply restrictions, leading to a decline in downstream innovation.
[3] For example, The National Transportation Safety Board determined that the 2016 fatal Tesla crash was partly due to the driver's inattention and overreliance on vehicle automation despite manufacturer safety warnings.